Over the years, MOKO has provided medical devices and associate services that have consistently reached our customer’s and applicable regulatory requirements bar. We have been continuously vetted through the stiff ISO13485 requirements and have proved ourselves worthy again and again for the certification.
What’s ISO13485 Certification
ISO13485 is the ultimate QMS (Quality Management System) standard to vet the eligibility of medical devices. Its main aim is to ensure medical products and related services are of the best quality to function optimally. Any company or organization that designs, develops, produces, services, stores, distributes or installs medical assets needs to meet these regulatory standards before deploying its services to consumers. As always, MOKO puts our customers’ interests first and have met these standards.
This certification is recognized worldwide. ISO13485 is currently the FDA’s mandatory Quality Management System after the U.S proposed an arrangement that harmonized ISO 13485:2016 with U.S. FDA 21 CFR 820. The ISO13485 certificate can be given to any registered organization, provided it meets the regulatory requirements no matter its size. However, the certification cannot be issued to one person since it’s not a personal standard.
Since the ISO13485 is not a set standard for products, it does not dictate medical products’ quality. Instead, it’s a product-based standard to be followed by targeted organizations.
ISO13485 Certification Process
Every organization dealing with medical devices must go through strict requirements. This is to make sure they fully comprehend the process needed to make and offer medical devices. MOKO was no exception. We underwent a thorough audit by a CB (Certified body) before we got our ISO 13485 certification, expected to run for three years. After three years, we’ll undergo another audit to get re-certified.
These requirements are updated regularly hence the need for re-certification after a period of time.
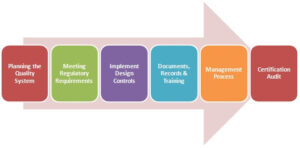
The certification process is a 6-steps journey. First, the organization needs to document and present its quality plants to implement changes in the QMS. Second, they must meet the regulatory requirement as per the region they are selling their products to. Third, they must implement design protocol as per their client’s needs. The Fourth step requires organizations to present their documentation for records and training in their quality management process. On the fifth step, the Certified Board evaluates the risk management process for the organization, which leads to the last step of the certification audit. This is done by special personnel from the medical sector and an ISO agent. Once the audit is complete, several corrective plans might be suggested, which the organization is supposed to accept so as to be issued the final ISO13485 certificate.
What ISO13485 Certification Means for Our Customers
It’s no secret that a medical device can strike the balance the life or death of a consumer. With that in mind, MOKO always strives to pass the quality management audit in the design, development and production processes to ensure the final product improves our consumers’ lives. This is why we’ve been ISO13485 certified over and over again.
Other than that, we’ve become UL, RoHS, ISO 14001 and ISO I9001 in an effort to better our overall operations for our customers.



























